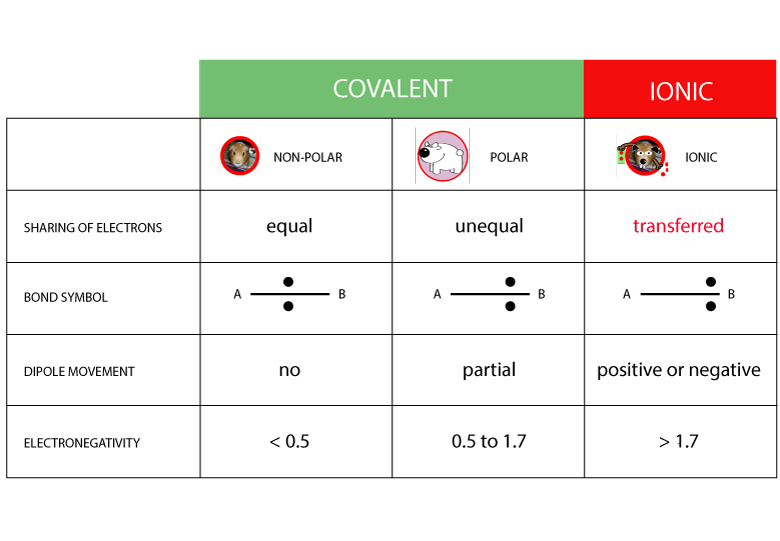
Is Magnesium Iodide Polar Or Nonpolar . It forms various hydrates mgi2·xh2o. The dipole moment measures the. magnesium iodide is an inorganic compound with the chemical formula mg i 2. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. In the magnesium iodide compound,. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Otherwise the molecule is said to be nonpolar.
from surfguppy.com
calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. In the magnesium iodide compound,. Otherwise the molecule is said to be nonpolar. magnesium iodide is an inorganic compound with the chemical formula mg i 2. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. It forms various hydrates mgi2·xh2o. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. The dipole moment measures the.
Comparision of Bonds Surfguppy Chemistry made easy for visual learners
Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. In the magnesium iodide compound,. Otherwise the molecule is said to be nonpolar. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. magnesium iodide is an inorganic compound with the chemical formula mg i 2. The dipole moment measures the. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); It forms various hydrates mgi2·xh2o. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); The dipole moment measures. Is Magnesium Iodide Polar Or Nonpolar.
From stock.adobe.com
Magnesium Iodide MgI2 molecule. Simple molecular formula consisting of Is Magnesium Iodide Polar Or Nonpolar if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); Otherwise the molecule is said to be nonpolar. In the magnesium iodide compound,. The dipole moment measures the. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. magnesium iodide. Is Magnesium Iodide Polar Or Nonpolar.
From slideplayer.com
Topic 1 Classification of Matter ppt download Is Magnesium Iodide Polar Or Nonpolar It forms various hydrates mgi2·xh2o. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); In the magnesium iodide compound,. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Otherwise the molecule is said to be nonpolar. magnesium iodide is a highly. Is Magnesium Iodide Polar Or Nonpolar.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Is Magnesium Iodide Polar Or Nonpolar Otherwise the molecule is said to be nonpolar. The dipole moment measures the. magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. In the magnesium iodide compound,. It forms various hydrates mgi2·xh2o. if such. Is Magnesium Iodide Polar Or Nonpolar.
From surfguppy.com
Comparision of Bonds Surfguppy Chemistry made easy for visual learners Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. The dipole moment measures the. if such. Is Magnesium Iodide Polar Or Nonpolar.
From chicfer.blogspot.com
orbital diagram magnesium Chicfer Is Magnesium Iodide Polar Or Nonpolar The dipole moment measures the. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. magnesium iodide is an inorganic compound with the chemical formula mg i 2.. Is Magnesium Iodide Polar Or Nonpolar.
From www.researchgate.net
(PDF) Molecular description for magnesium iodide Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. Otherwise the molecule is said to be nonpolar. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and. Is Magnesium Iodide Polar Or Nonpolar.
From www.pinterest.fr
Is HI Polar or NonPolar? (Hydrogen Iodide) Hydrogen iodide, Iodide Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. The dipole moment measures the. magnesium iodide is an inorganic compound with the chemical formula mg i 2. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); In the magnesium. Is Magnesium Iodide Polar Or Nonpolar.
From ar.inspiredpencil.com
Magnesium Cycle Is Magnesium Iodide Polar Or Nonpolar The dipole moment measures the. It forms various hydrates mgi2·xh2o. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In the magnesium iodide compound,. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. calculate the electronegativity difference (δen) and. Is Magnesium Iodide Polar Or Nonpolar.
From learningschoolbiey2w.z14.web.core.windows.net
Polarity Of Molecules Physical Science Ppt Is Magnesium Iodide Polar Or Nonpolar It forms various hydrates mgi2·xh2o. The dipole moment measures the. magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. In the magnesium iodide compound,. magnesium iodide is an ionic compound formed by the combination. Is Magnesium Iodide Polar Or Nonpolar.
From brainly.in
what is the symbol of magnesium iodide Brainly.in Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. magnesium iodide is an inorganic compound with the chemical formula mg i 2. The dipole moment measures the. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the.. Is Magnesium Iodide Polar Or Nonpolar.
From brainly.com
The diagram shows how magnesium and iodine atoms form magnesium iodide Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. calculate the. Is Magnesium Iodide Polar Or Nonpolar.
From techiescientist.com
Is I2 Polar or Nonpolar? Techiescientist Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. magnesium iodide is an inorganic compound with the chemical formula mg i 2. The dipole moment measures the. magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In the magnesium. Is Magnesium Iodide Polar Or Nonpolar.
From www.masterorganicchemistry.com
The three types of nucleophiles you meet in organic chemistry — Master Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is an inorganic compound with the chemical formula mg i 2. Otherwise the molecule is said to be nonpolar. The dipole moment measures the. In the magnesium iodide compound,. calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. magnesium iodide is an ionic compound. Is Magnesium Iodide Polar Or Nonpolar.
From www.youtube.com
Is MgF2 (Magnesium fluoride) Ionic or Covalent? YouTube Is Magnesium Iodide Polar Or Nonpolar magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Otherwise the molecule is said to be nonpolar. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. magnesium iodide is an inorganic compound with the chemical formula mg i 2.. Is Magnesium Iodide Polar Or Nonpolar.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Is Magnesium Iodide Polar Or Nonpolar if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. The dipole moment measures the. Otherwise the molecule is said to be nonpolar. In the magnesium iodide compound,. magnesium iodide. Is Magnesium Iodide Polar Or Nonpolar.
From stock.adobe.com
Magnesium iodide mgi2 molecule. Simple molecular formula consisting of Is Magnesium Iodide Polar Or Nonpolar calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. The dipole moment measures the. Otherwise the molecule is said to be nonpolar. It forms various hydrates mgi2·xh2o. . Is Magnesium Iodide Polar Or Nonpolar.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Is Magnesium Iodide Polar Or Nonpolar Otherwise the molecule is said to be nonpolar. The dipole moment measures the. if such a charge separation exists, the molecule is said to be a polar molecule (or dipole); magnesium iodide is an inorganic compound with the chemical formula mg i 2. magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon. Is Magnesium Iodide Polar Or Nonpolar.